(A) Emitting an
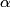 particle would reduce the atomic mass by particle would reduce the atomic mass by 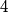 units and the atomic number by units and the atomic number by 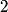 units. Therefore, this is not correct. units. Therefore, this is not correct. (B) Emitting an electron only would give the atom a net positive charge. Therefore, this is not correct. (C) Emitting a neutron only would reduce the atomic mass by 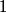 unit and keep the atomic number unchanged. Therefore, this is not correct. unit and keep the atomic number unchanged. Therefore, this is not correct. (D) Emitting a positron only would give the atom a net negative charge. (E) Electron capture by the nucleus means that a proton in the nucleus combines with an electron in the atom to form a proton. The neutrino (which is actually an electron neutrino) is emitted in order to conserve electron number. This is consistent with the end product of lithium- 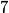 . Therefore, this answer is correct. . Therefore, this answer is correct. |